July 2015
Mesothelioma Treatment, Eph-B4, is now in Expanded Clinical Trials at USC/Norris under Dr. Gill supervision
October 2014
Mesothelioma Eph-B4 Treatment Submitted to FDA for Phase I Clinical Trial
Malignant pleural mesothelioma often develops decades following exposure to asbestos. Currently, the best therapy produces a response in only half of the patients, and the median survival with the current therapy remains under a year. A search for novel targets and therapeutics is underway, and recently identified therapeutics includes Eph-B4.
The mesothelioma research lab at USC/Norris Comprehensive Cancer Center, where our donations go, has investigated the use of Eph-B4 in human mesothelioma tissues by immunohistochemistry. Xenograft tumors established with human mesothelioma cells were treated with an Eph-B4 inhibitor, as well as with a combinatorial effect of Eph-B4 and a biologic agent.
Eph-B4 was overexpressed, or shrunk the size of the tumor, in 72% of the mesothelioma tissues evaluated, with 85% of epithelioid and 38% of sarcomatoid subtypes demonstrating overexpression. The Eph-B4 inhibitor was highly active as a single agent to inhibit tumor growth and the combination of Ep-B4 and a biologic agent, Bevacizumab, was superior to each agent alone and led to complete tumor regression!
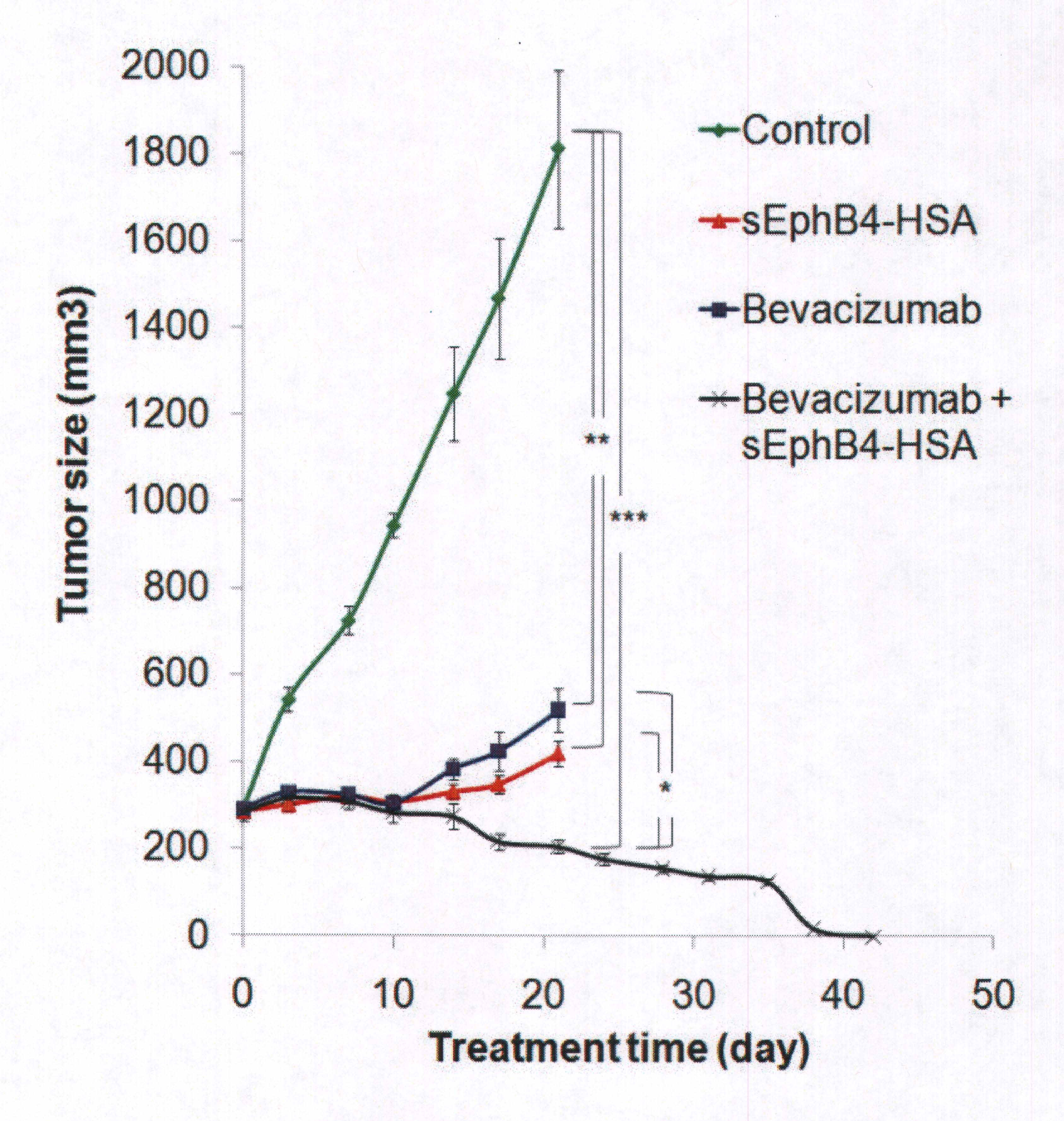
Since the study, Eph-B4 has been submitted to the FDA for Phase I Clinical Trials and is currently being offered to patients at the USC/Norris Comprehensive Cancer Center. Doctors from facilities such as, the University of Chicago , University of California , Davis , University of California , San Diego and Georgetown University , want to work with USC/Norris in treating more patients by offering their patients Eph-B4.
EPH-B4 Clinical Trial
EPH-B4 is a new drug created by Dr. Parkash Gill.. EPH-B4 is a drug that promises much potential in the fight against a variety of cancers and possibly a cure.
October 1, 2012
Dr. Parkash Gill of the USC Norris Comprehensive Cancer Center has announced that the new cancer fighting drug EPH-B4 has been submitted to the FDA for Phase I Clinical Trials and is available to qualifying mesothelioma patients. In the initial Phase I Trial EPH-B4 is available to newly diagnosed patients who have not undergone any conventional treatment options and to those patients who have exhausted all other treatment options such as surgery and chemotherapy. This is truly exciting as Dr. Gill has reported that EPH-B4 has shown great promise as a solo treatment or in conjunction with other drugs as Alimta, Cisplatin and Carboplatin.
About Clinical Trials
Clinical trials are separated into four distinct phases:
Phase I & II: the first two phases of any clinical trial revolve around drug safety. Candidates for phases I & II include patients with an advanced form of a given disease (in this case, cancer), who are given the opportunity to try experimental treatments.
Phase III: the third phase of any clinical trial revolves around comparing the drug with standard forms of treatment proven to work. Participants of phase III have a more optimistic prognosis while also meeting clinical trial criteria.
Phase IV: the fourth and final phase of any clinical trial coincides with the preliminary marketing of the drug. Studies gather information on the drug's effect in various populations while monitoring any long-term side effects.
To determine if you qualify for the EPH-B4 studies, please consider contacting Elizabeth Paul, Executive Director, by calling 1-800-909-MESO (6376), or use this form to Ask Dr. Gill about the trials.
Also, consider going to our FaceBook page:
to obtain a FREE copy of the eBook: "10 Easy Steps to Find and Choose a Clinical Trial."


For questions related to the foundation and to make contributions please contact:
Executive Director
Toll Free:
(800) 909-Meso (6376)
3011 Townsgate Rd, Suite 450
Westlake Village, CA 91361
For more information and other questions contact:
(800) 909-6376
©2016 Mesothelioma Research Foundation Of America

